Federal Judge Plans JUUL Bellwether Case Selection Process

U.S. District Judge William H. Orrick III, overseeing the federal JUUL multidistrict litigation (MDL), issued an order establishing the method for the parties to choose the cases that will be prepared for the first bellwether trials, which is set to begin in early 2022.
JUUL is the most popular e-cigarette in the U.S. manufactured by Juul Labs, Inc., an American electronic cigarette company. The company holds 97 percent of the e-cigarette market, and the rest is covered by Vuse, MarkTen, Blu, and Logic. Around 75 percent of all e-cigarettes sold in the U.S. are manufactured by Juul. The company entered the market in 2015, and as per the Centers for Disease Control and Prevention (CDC), each JUUL pod contains a nicotine level of 20 regular cigarettes, which is higher than other e-cigarettes. JUUL is popular among teens and young adults due to its sleek design and nicotine level. Due to its dominance, the term Juuling is also popular among users.
JUUL, an e-cigarette, has been in news over its popularity among young users and for the growing number of lawsuits alleging that it leads to serious health problems, such as severe lung injuries, seizures, nicotine addiction and poisoning, and an increased risk of heart attacks and strokes. The rise resulted in the formation of an MDL 2913, and the lawsuits are currently centralized in the U.S. District Court for the Northern District of California, where JUUL Labs, Inc. is headquartered.
According to the order dated September 9, Judge Orrick indicated that he is considering the plaintiffs’ suggestions in bellwether case selection and case schedule process. The parties and the court will select a total of 24 cases, of which six will be selected by plaintiffs and six by the defendant. The remaining twelve will be randomly selected cases by the court for the initial bellwether discovery pool, which would be done by December 15, 2020.
The core discovery process, post the selection, will conclude by April 15, 2021. By April 22, the parties will be allowed to strike one case each from the pool, and on April 30, the parties will identify eight cases, four each, from the remaining pool.
The court will then select individual cases, and a series of five bellwether trials are expected to begin on February 22, March 28, May 9, June 20, and August 1, 2022.
Apart from individual addiction claims, several class action lawsuits have also been filed by school districts and other entities who suffered damages from the vaping epidemic. The lawsuits allege that the manufacturer created false and misleading advertisements for JUUL, plaguing the U.S. in recent years.
SSRIs Linked To Risk Of Type 2 Diabetes In Children

JAMA Psychiatry published a study earlier this month, indicating that children who were prescribed selective serotonin reuptake inhibitor (SSRI) antidepressants, like Paxil, Celexa, or Zoloft are at a higher risk of developing type 2 diabetes.
Selective serotonin reuptake inhibitors (SSRIs) are typically used in the treatment of major depressive disorder and anxiety disorders that work by altering the levels of a mood-enhancing chemical called serotonin. The first SSRI introduced in the market was fluoxetine in 1985. SSRI is indicated in the treatment of major depression, anxiety disorder, panic disorder, social anxiety disorder, social phobia, bulimia nervosa, obsessive-compulsive disorder, personality disorder, premenstrual disorder, and posttraumatic panic disorders. Off-label use of SSRI is a migraine, diabetic neuropathy, neurocardiogenic syncope, and fibromyalgia.
The national pediatric study conducted by Harvard researchers involved more than 1.5 million publicly and privately insured patients, aged between 10 to 19 years old, who were taking SSRIs as a treatment for depression. As per the findings, a 13% increased risk of type 2 diabetes was linked in publicly insured adolescents treated with SSRIs as compared to untreated patients. A 33% increased risk was seen in teenagers who continuously used SSRIs, filling one or more prescriptions every three months.
The study also implies that there might be 6.6 additional cases of diabetes per 10,000 adolescent or teen patients treated with SSRIs for the past two years, and the risk factor was statistically insignificant for privately insured young patients.
Last month, the Massachusetts General Hospital researchers also published a study in the medical journal JAMA Neurology, indicating that the antidepressants, prescribed to individuals after suffering a stroke, may increase the risk of suffering a brain bleed later.
The use of SSRIs is growing as more and more adolescents are suffering from depression and other anxiety disorders. Previous studies have indicated the link between SSRI antidepressants and diabetes among adults; children and teens are usually excluded from clinical trials, but concerns were always raised.
The researchers concluded that children and adolescents receiving SSRI treatment might be at a small increased risk of developing type 2 diabetes, and the potential small risk should be viewed in relation to the effectiveness of the antidepressants.
Plaintiffs Seek To Consolidate Elmiron Lawsuits
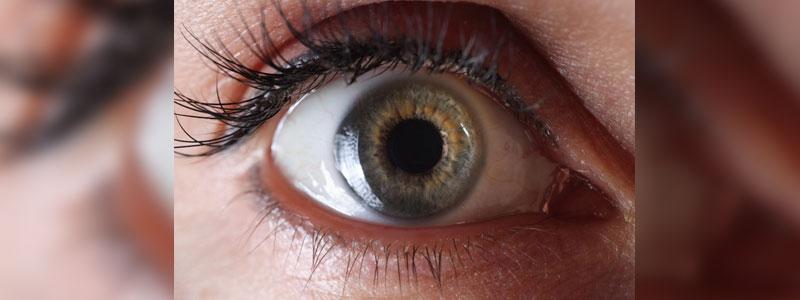
On September 23, two plaintiffs who claim that the bladder medication, Elmiron, results in permanent vision problems have asked the U.S. Judicial Panel on Multidistrict Litigation (JPML) to consolidate and centralize the cases before U.S. District Judge Brian R. Martinotti in the District of New Jersey.
Elmiron, also known by its generic name pentosan polysulfate sodium, or PPS, is an oral prescription drug that is used to treat pain/discomfort caused by bladder disorder interstitial cystitis, or IC.
The drug is manufactured by Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson, and was approved by the U.S. Food & Drug Administration (FDA) in 1996. The drug is approved under the Orphan Drug Act or ODA, which gives special status and incentives to sponsors, or manufacturers, of medications that treat rare diseases.
The plaintiffs filed the motion to transfer over the rising number of claims being filed in different U.S. District Courts. The plaintiffs are seeking centralization for coordinated pretrial proceedings and to avoid conflicting pretrial rulings from different courts, avoid duplicative discovery, and serve the convenience of common witnesses, parties, and the judicial system.
The motion has also noted some very similar, if not virtually identical, allegations that the manufacturer is facing.
-
Elmiron can cause retinal pigmentary changes and/or maculopathy as supported by, among other things, the growing medical literature.
-
Defendants negligently created, designed, researched, developed, manufactured, tested, marketed, advertised, promoted, distributed, and sold Elmiron to the public, including the plaintiffs in the respective actions, and caused their alleged injuries.
-
Defendants knew or should have known of the dangers and defects associated with Elmiron.
-
Defendants failed to warn of the dangers and defects associated with Elmiron.
-
All plaintiffs suffered grave ocular injuries as a result of using the defendant’s defective Elmiron.
Currently, 24 civil actions related to Elmiron are pending before Judge Martinotti, and 39 additional personal injury actions are filed in 10 different federal courts across the country.
The defendant has not yet responded to the motion, and a hearing session is scheduled for December 3, 2020, in San Antonio, Texas, to hear the oral arguments from various parties involved in the lawsuits.
