Nationwide Class Over Elmiron Vision Monitoring Cases Denied

Earlier this month, U.S. District Judge Wendy Beetlestone issued an opinion, stating that claims filed seeking vision monitoring program by the former users of Elmiron residing in the U.S. cannot be litigated as a nationwide class action.
The lawsuit was filed by a plaintiff who was prescribed Elmiron to treat his interstitial cystitis (IC). The plaintiff, on behalf of other IC drug users, whom he seeks to represent as a class, alleged that the defendants negligently failed to conduct adequate safety testing, notify the Federal Drug Administration (FDA) of the link between Elmiron and maculopathy, and alert consumers to the risks of taking the drug.
The plaintiff demanded the defendants to pay for ongoing medical monitoring of prospective class members on the basis that early diagnosis achieved through a monitoring regime will lead to benefits in treatment, management, rehabilitation, or mitigation of long-term health consequences.
The Amended Complaint set forth three separate putative classes: the “Proposed Illinois Class,” the “Proposed Pennsylvania Class,” and “the Proposed Nationwide Class.”
The defendants, Johnson & Johnson (J&J) and its subsidiary Janssen Pharmaceuticals, Inc., filed a motion to strike only "the Proposed Nationwide Class," contending that there are laws in some states, which prevent such medical monitoring claims where there has been no diagnosed injury.
Judge Beetlestone agreed to strike the claims seeking medical monitoring over the medication filed in the Eastern District of Pennsylvania and allowed other claims to proceed in the litigation.
A hearing session is scheduled for December 3, 2020, in San Antonio, Texas, to hear the oral arguments from various parties involved in the lawsuits over the consolidation request filed by a group of plaintiffs asking the U.S. Judicial Panel on Multidistrict Litigation (JPML) to centralize the cases before U.S. District Judge Brian R. Martinotti in New Jersey.
Biomet Loses In Two Separate M2a Magnum Hip Implant Trials
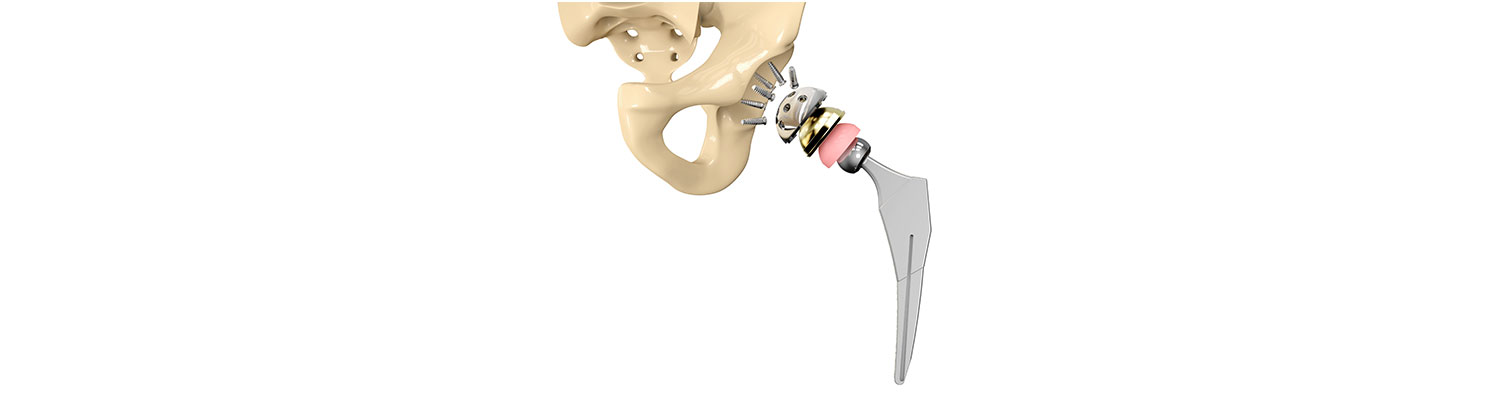
On Monday, the U.S. District Court for the Northern District of Iowa entered a judgment of $3.55 million verdict in favor of an Iowa woman over claims alleging that Biomet’s metal-on-metal M2a Magnum hip implant system was defectively designed.
According to the lawsuit filed, the plaintiff from Fort Dodge, Iowa, had her left hip replaced with Biomet M2a Magnum in July 2007. The plaintiff started experiencing pain as the hip loosened over time, and the level of chromium in her body also increased. The plaintiff underwent revision surgery in June 2012.
The trial started on November 09 with eight jurors, out of which two were dismissed due to COVID-19-related problems. Six jurors gave the final verdict, which was signed on Friday and made public on Monday.
The federal jury in Cedar Rapids, Iowa, awarded $1.05 million in compensatory damages and $2.5 million in punitive damages to the plaintiff and her husband. The compensatory damages included $500,000 for past physical and mental pain and suffering, $250,000 for future pain and suffering, $100,000 for past loss of function of body and mind, and $200,000 for future loss of function.
The case is considered "virtually identical" to a trial that concluded in Missouri, where the state's federal jury awarded $21 million to a plaintiff and her husband and refused to impose punitive damages against Biomet Inc.
Judge Stephen R. Clark of the U.S. District Court for the Eastern District of Missouri signed the jury's judgment on Monday, which follows Biomet Inc.'s failed attempt for mistrial in the punitive damages phase of the M2a Magnum hip implant lawsuit, which the manufacturer had pushed for reconsideration after one juror contracted COVID-19.
According to the order, which was made public on Tuesday, the verdict includes $20 million to the plaintiff, plus post-judgment interest and costs of the action, and $1 million to her husband, plus post-judgment interests and costs of the action.
OxyContin Maker Pleads Guilty Over Three Felony Offenses

On Tuesday, OxyContin manufacturer Purdue Pharma LP CEO and Chairman pleaded guilty on behalf of the company to three-count felony offenses, which include one count of dual-object conspiracy to defraud the United States and to violate the Food, Drug, and Cosmetic Act, and two counts of conspiracy to violate the Federal Anti-Kickback Statute.
The top officers faced a series of questions in a teleconference proceeding from Assistant U.S. Attorney J. Stephen Ferketic over the company's knowledge, conduct, and offenses dating to 2007.
During the hourlong plea hearing convened in New Jersey federal court by U.S. District Judge Madeline Cox Arleo, the company admitted that it made payments to two doctors through Purdue’s doctor speaker program to induce those doctors to write more opioid prescriptions. The company also acknowledged that it made payments to Practice Fusion Inc., an electronic health records company, in exchange for referring, recommending, and arranging for the ordering of Purdue’s extended-release opioid products – OxyContin, Butrans, and Hysingla.
According to the terms of the plea agreement, the company will pay penalties considered to be the largest ever levied against a pharmaceutical manufacturer. The penalties include a criminal fine of $3.544 billion and an additional $2 billion in criminal forfeiture, of which the company will pay $225 million within three business days following the entry of a judgment of conviction in accordance with the Plea Agreement.
The company also agreed to a civil settlement of $2.8 billion to resolve its civil liability under the False Claims Act, and the Sackler family agreed to pay $225 million in damages to resolve its civil FCA violations liability.
Last week, the bankruptcy court in the Southern District of New York approved the financial terms of the global resolution with the company. According to the resolution, the company will close down, and a new "public benefit company (PBC)" will be formed using its assets. The newly formed company will be controlled by a trust or similar entity meant for the benefit of the nation's public. The future earnings of the company will be used to compensate for the fines and penalties. Based on PBC's potential value to state and local governments, the U.S. Department of Justice (DOJ) said that it is willing to credit up to $1.775 billion against the agreed $2 billion forfeiture amount.
Magris Resources Acquires Imerys Talc For $223M

Imerys Talc America had declared bankruptcy last year following the rise in talcum powder lawsuits and U.S. District Judge Freda Wolfson's ruling to proceed with the lawsuit against Johnson & Johnson (J&J) to whom it supplied talc.
A Toronto, Ontario-based company, Magris Resources Canada, Inc., has agreed to buy the assets of Imerys' North American Talc Subsidiaries for $223 million.
Currently, J&J, Imerys, and other defendants face nearly 20,000 Baby Powder and Shower-to-shower lawsuits. The allegations include that the companies failed to warn the talcum users about the side effects of applying the products around the genitals.
Imerys faced allegations for withholding the information of risks associated with talc and asbestos particles present in the products. This followed with heavy penalties resulting in Imerys declaring bankruptcy to avoid further escalations.
Magris Resources Canada, Inc., successfully made the bid to acquire Imerys after the U.S. Bankruptcy Court for the District of Delaware announced its sale.
Imerys officials said that the sale would not happen until the first quarter of 2021, and a hearing has been scheduled for November 25 in a Canadian court for the approval of the deal.
The sale will eventually settle the charges against Imerys in the litigations as it will provide funds to deal with the ovarian cancer settlements.
Currently, all the talc powder lawsuits are centralized before Judge Freda L. Wolfson in the District of New Jersey for pretrial proceedings and coordinated discovery as part of an MDL. The litigation trials may have a substantial impact on settlement negotiations if J&J fails to defend the trials.
Study Links Glyphosate To Endocrine Disrupting Chemicals

Last month, a study was published in the peer-reviewed scientific journal Chemosphere in which Chilean researchers stated that glyphosate, the active component of the most commonly used herbicide, Roundup, meets at least 8 key characteristics (KCs) of an endocrine-disrupting chemical (EDC).
EDC also referred to as endocrine disruptors, hormonally active agents, or endocrine-disrupting compounds, interferes with the hormonal system and causes cancerous tumors, birth defects, and other developmental disorders.
The World Health Organization’s International Agency for Research on Cancer (IARC) classified glyphosate as a probable carcinogen in March 2015. Since then, the concerns associated with the risk of non-Hodgkin's lymphoma (NHL) and certain other cancers has been growing among its users.
In the recent study, the researchers compared the properties of glyphosate to 10 KCs of guidelines proposed in the expert consensus statement published in 2020 that help to classify endocrine disruptors. The researchers concluded that although 8 KCs were satisfied, prospective cohort studies are further required to clarify whether glyphosate is an EDC.
In the legal world, attorneys representing Bayer filed a joint Case Management Statement and Litigation Plan earlier this month, indicating that the company missed the deadline set by the court to settle thousands of Roundup lawsuits.
The company also issued a third-quarter report in the same week, indicating a challenging quarter impacted substantially by the Covid-19 pandemic and the legal action over Roundup.
Currently, Bayer is facing approximately 125,000 filed and unfiled claims. The lawsuits are presided by U.S. District Judge Vince Chhabria under MDL No. 2741 in the U.S. District Court for the Northern District of California.
