Talcum Powder MDL Status Conference To Be Held Today

Last week, parties involved in the talcum powder litigation submitted a Status Report and Proposed Joint Agenda for the status conference scheduled for today to review the progress of the bellwether cases.
The report indicates the development made in Stage One and Stage Two cases, which will be reviewed today by U.S. District Judge Freda L. Wolfson, overseeing the talcum powder litigation docket. Judge Wolfson will also address the concerns raised by the parties over the case selection process and a schedule for final expert discovery in those claims.
In June, Judge Wolfson announced a list of randomly selected 1,000 talcum powder cancer lawsuits for bellwether discovery procedures. In September, the pool was further reduced to a group of about 30 claims, which included ten selected by each party, along with ten selected by the court.
The result of these bellwether trials will not bind on other claims in the docket but will impact the settlement negotiations that the defendant may involve in to avoid future bellwether claims.
Earlier this year, Johnson & Johnson (J&J) stopped selling its talcum-based products in the U.S. and Canada, claiming declined consumer demand and misinformation about the safety of the products.
Johnson's Baby Powder, one of the most popular products containing talcum powder, is linked to increasing a woman's risk of ovarian cancer if she uses it regularly in the genital area. In a few cases, the cancer tissue was studied using an electron microscope and was found to have talc in it, which supported the claim that the cancer was caused by the body powder and increases the talc-related cancer risk.
Allegations include that the company knew about the risks of asbestos exposure from the company’s talc-based products causes ovarian cancer or mesothelioma, yet failed to warn the public. Lawsuits also claim J&J hid the fact that talcum powder contains asbestos.
Currently, J&J is facing more than 20,000 Baby Powder and Shower-to-Shower lawsuits, each raising similar allegations about the presence of asbestos and the risk of cancer. Most of the claims are consolidated under MDL No.: 2738, whereas some are pending in state courts in Pennsylvania. Cases are also pending in a California coordinated proceeding as a part of Judicial Council Coordinated Proceeding No. 4877.
J&J's Appeal Over $325M Asbestos Talc Verdict Rejected

On November 12, a stipulation was filed in the New York Supreme Court for New York County, indicating that the court has rejected Johnson & Johnson’s (J&J) challenge over a $325 million asbestos talc verdict, stating there was legally sufficient evidence to support the liability.
However, the state's trial court agreed to the company's assertion that the compensatory and damages award was excessive and ordered a new trial on damages unless the plaintiffs stipulated to reduce the award.
The lawsuit was filed by a couple in 2017, claiming that J&J's baby powder and scented Shower to Shower products resulted in the woman's pleural mesothelioma, a cancer of the lining of the lungs. The company was hit with $300 million in punitive damages in 2019 after the jury found it liable to the plaintiffs’ defective design claim and failure-to-warn claim.
In the initial verdict, the couple was granted $25 million in compensatory damages apart from the $300 million in punitive damages. The case will undergo a new trial unless the plaintiffs set down to reduce the award to $120 million, which would include $10 million for past and $3.5 million for future pain and suffering, $1.2 million for loss of companionship and services, and $300,000 for future pain and suffering, along with $105 million in punitive damages.
Johnson's Baby Powder, one of the most popular products containing talcum powder, is linked to increasing a woman's risk of ovarian cancer if she uses it regularly in the genital area. In a few cases, the cancer tissue was studied using an electron microscope and was found to have talc in it, which supported the claim that the cancer was caused by the body powder and increases the talc-related cancer risk.
Allegations include that the company knew about the risks of asbestos exposure from the company’s talc-based products causes ovarian cancer or mesothelioma, yet failed to warn the public. Lawsuits also claim J&J hid the fact that talcum powder contains asbestos.
Bellwether trials against J&J over its more than 20,000 Baby Powder and Shower-to-Shower lawsuits are also proceeding, and a status conference was held on November 17 to review the progress of the cases.
Teva Moves A ParaGard IUD Lawsuit To Federal Court System

Earlier this week, Teva Pharmaceuticals filed a notice over the removal of a ParaGard IUD lawsuit to the Eastern District of Pennsylvania, which was originally filed in the Philadelphia County Court of Common Pleas.
The lawsuit was filed by a North Carolina woman in October 2020, alleging that the birth control device fractured during removal, leaving her with permanent injuries.
According to the court memorandum, the plaintiff had the device implanted by a physician on October 14, 2013, and during the removal on August 14, 2017, one arm of the device remained inside her body. Two unsuccessful attempts were made on September 21, 2017, and May 29, 2018, and the physician was unable to retrieve the broken pieces.
The plaintiff, in her complaint, asserted that the device was marketed as safe and effective, and the defendants knew or should have known that it was defective and unreasonably dangerous. She further claimed that the warnings were vague, incomplete, or otherwise wholly inadequate to alert prescribing physicians and patients to the actual risks associated with the device.
The defendants removed the lawsuit from state court to federal court due to diversity jurisdiction, as the plaintiff is from North Carolina and Teva Pharmaceuticals is based in New Jersey.
A hearing session is scheduled for December 3, 2020, in San Antonio, Texas, to hear the oral arguments from various parties over the motion filed by the plaintiffs asking the U.S. Judicial Panel on Multidistrict Litigation (JPML) to consolidate all ParaGard IUD cases before one judge in the U.S. District Court for the Central District of California.
ParaGard, a small IUD (intrauterine device) that is used to prevent pregnancy works differently using one simple active ingredient, copper, instead of hormones, unlike its other counterparts. It is a T-shaped device and can help avoid pregnancy for up to 10 years.
The copper present in the device is continuously released into the uterine cavity, which interferes with sperm transport or fertilization, and the prevention of implantation. The use of IUD results in less than 1 pregnancy per 100 women each year as per the pregnancy rate in clinical studies. The FDA approved five IUD brands for sale in the U.S.: Mirena, Kyleena, Liletta, Skyla, and ParaGuard.
Biomet's Motion For Mistrial Over Punitive Damages Denied
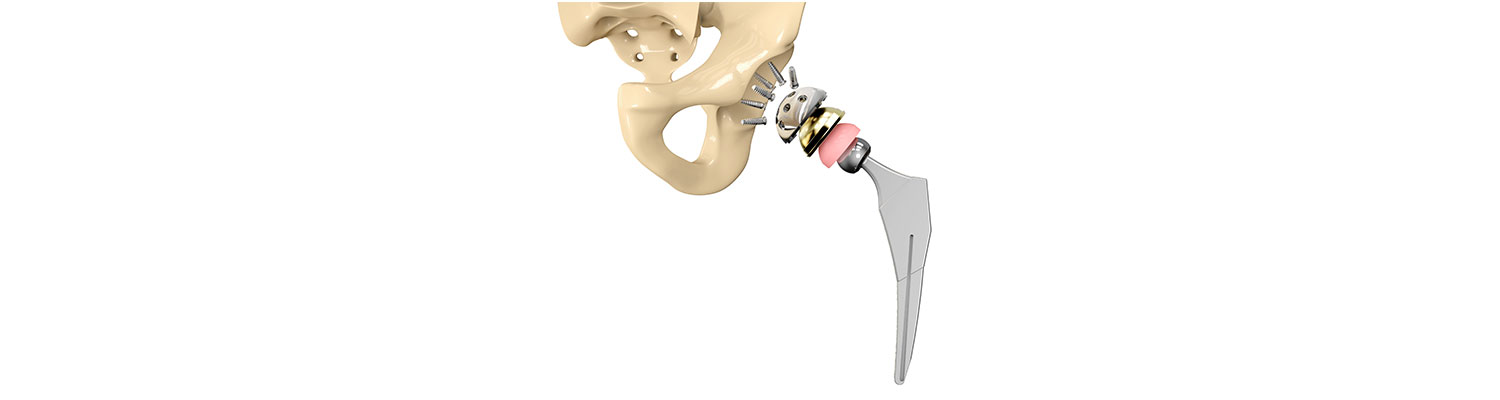
On Wednesday, U.S. District Judge Stephen Clark of the U.S. District Court for the Eastern District of Missouri rejected Biomet Inc.'s motion for mistrial in the punitive damages phase of an M2a Magnum hip implant lawsuit, which the manufacturer had pushed for reconsideration after one juror contracted COVID-19.
According to the court documents, the punitive damages phase concluded the same day. However, the verdict was not made publicly available.
The plaintiff involved in the lawsuit had both her hips replaced with Biomet's M2a Magnum in early 2008. The plaintiff started experiencing pain in her left hip in 2010, which resulted in her first revision surgery in March 2011. A lawsuit was filed in 2013, alleging that the hip implant was defective and the manufacturer should have known about it as the design was based on an earlier design, called the M2a Taper, which had allegedly caused problems previously.
The lawsuit went to trial last month, and a compensatory damages verdict was concluded in favor of the plaintiff on October 22.
Following the verdict, attorneys representing the defendant asked the court to reconsider the compensatory-damages verdict, stating that one juror tested positive for the COVID-19 test during the earlier phase. The defendant again pushed for a mistrial on Wednesday, arguing that trying the second phase to a different jury after the removal of one juror violated the 7th Amendment rights.
The federal judge ruled against the defendant's motion for a continuance or mistrial, and the reasoning of the order was not promptly clear.
Zimmer Holdings is an Indiana-based orthopedics company involved in providing hip and knee solutions including joint and skeletal replacement systems and surgical tools as well as biologics designed to assist in joint replacement surgeries. In April 2014, Zimmer and Biomet announced to come together to provide joint replacement, bone repair, and dental implant solutions. In June 2015, they started out as Zimmer Biomet and began offering solutions to the musculoskeletal healthcare industry. The hip and knee implants were intended to relieve pain, provide good quality of life to the patients. However, Zimmer lawsuits filed by several individuals claimed the metal-on-metal articulation used in the system resulted in Metallosis, because of the metal grounding on metal as it leads to toxic metal shards being released into the patient's surrounding tissues and bone
