FDA Issues A Warning Letter To Allergan Over Breast Implant
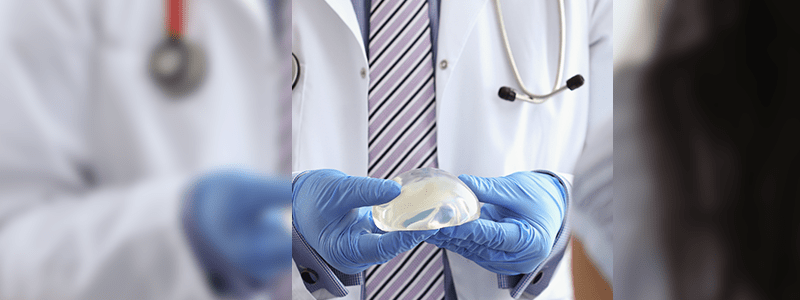
The U.S Food & Drug Administration (FDA) has issued a warning letter to Allergan, Inc., as the company failed to conduct post-approval studies on two different breast implant models, which were recalled last year over allegations that they might expose women to a rare form of cancer known as breast implant-associated anaplastic large cell lymphoma (BIA-ALCL).
Breast implants are used in both breast augmentation surgery (to increase the breast size) and in breast reconstruction (to replace breast tissue that has been removed due to cancer or trauma or that has failed to develop properly due to a severe breast abnormality).
Allergan Inc.’s breast implants include saline and silicone devices used in breast augmentation and reconstruction. The company is one of the largest breast implant manufacturers in the world and started selling breast implants in 2006. Allergan’s flagship breast implant brand is Natrelle, but it also sells implants under its subsidiaries Inamed and McGhan. The manufacturer received approval from the FDA on 11/17/2006.
On May 14, the FDA issued a warning letter to Allergan as the studies on the recalled breast implants were necessary to be completed to assess potential long-term outcomes among women who are already implanted with the devices. Natrelle Silicone-Filled (Round Responsive) Breast Implants and Natrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled Breast Implants are the two Allergan products that required post-approval assessment.
Last year, all the breast implant products that featured the macro textured design used in Natrelle implants were recalled by Allergan as ordered by the federal regulators in the United States. The recall was announced by the FDA as all the reported cases of BIA-ALCL were linked to the design of the product.
The warning letter issued by the FDA indicated that the company agreed to conduct the study in October 2006 but failed to achieve it in the coming years. The company even failed to collect crucial data on safety endpoints such as long-term complications, connective tissue diseases, neurological effects, reproductive issues, lactation, suicide, and other issues, including cancer. Allergan was even supposed to collect complication data from physician evaluations on the product's completion of 1, 4, and 10 years.
Allergan must respond to the letter within 15 days by providing a relevant plan to address the issues and violations. Apart from Allergan, even Ideal Implant Incorporated received a warning letter from the FDA over the same issue.
Currently, Allergan faces at least 50 lawsuits over breast implant cancer problems, which may increase gradually. All the breast implant lawsuits have been consolidated to form multidistrict litigation (MDL) before the U.S. District Judge Brian R. Martinotti in the District of New Jersey as it will prevent duplicative discovery and will result in a common outcome of the trials.
Imerys Talc Will Sell Its Subsidiaries To Settle Talcum Lawsuits
Imerys Talc, talc supplier of Johnson & Johnson will sell off its several subsidiaries to pay the settlement amount of thousands of talcum powder lawsuits filed by women nationwide.
Johnson's Baby Powder, one of the most popular products containing talcum powder, is linked to increasing a woman's risk of ovarian cancer if she uses it regularly in the genital area. In a few cases, the cancer tissue was studied using an electron microscope and was found to have talc in it, which supported the claim that the cancer was caused by the body powder and increases the talc-related cancer risk.
According to a press release (PDF) issued by Imerys Talc on May 15, the company will auction Imerys Talc America, Imerys Talc Vermont, and Imerys Talc Canada units to pay the settlement amount that will go to a settlement fund, and it will be used to compensate the consumers who have claimed of developing ovarian cancer or other injuries linked to talc ingredients.
The settlement will release Imerys Talc from the involvement in nearly 20,000 Baby Powder lawsuits and Shower-to-Shower lawsuits throughout the federal court system, but Johnson & Johnson will continue to face the trials over failing to warn the users that its talc-based products may contain asbestos fibers that can cause cancer. Details of the amount of the settlement are not yet known.
All the talcum powder lawsuits are centralized before Judge Freda L. Wolfson in the District of New Jersey, as part of an MDL, or multidistrict litigation for a common outcome.
The defense lawyers for Johnson & Johnson argue that plaintiffs’ expert witness testimony is not sufficiently reliable under the federal Daubert standard to prove that the use of talcum powder causes cancer. However, Judge Wolfson cleared the way for the talc lawsuits to proceed and indicated the experts to provide their opinions to the juries.
If the bellwether trials fail to settle the claims of the plaintiffs against Johnson & Johnson, then all the cases will be sent back to their respective District Courts nationwide for individual trials.
J&J To Discontinue Talc Sales In The U.S. & Canada

Johnson & Johnson (J&J) has decided to stop selling its talcum products in the U.S. and Canada due to declined consumer demand and misinformation about the safety of the products.
According to a report published on the official website of J&J, the company terminated the shipments of hundreds of items in the U.S. and Canada in March to evaluate its inventory amidst the coronavirus pandemic. Since March, J&J has maintained social distancing in its facilities and focused on shipping products with high demand. The company faces thousands of lawsuits that claim to cause mesothelioma and ovarian cancer because of its talc-based products.
The spokesperson for J&J told the company will defend its products as scientific studies conducted by the medical experts over the decades ensure its product safety.
According to J&J, the sale of the products will continue through the retailers until the inventory is drained off meanwhile, cornstarch-based baby powder will be available alongside.
Ted Meadows of Beasley Allen law firm is the lawyer for the plaintiffs suing J&J. He said that J&J should have removed Johnson's Baby Powder from the market decades ago and should now accept the responsibility for the cause of cancer among the thousands of women who are suffering or who have died as a result of ovarian cancer due to the talcum powder products. J&J recalled a shipment in October as the U.S. Food and Drug Administration found asbestos during an inspection.
All the talcum powder lawsuits are centralized before the U.S District Judge of New Jersey Freda L. Wolfson, who considered plaintiffs' expert testimony under the federal Daubert standard and opened a path to trials in the multidistrict litigation.
Johnson's Baby Powder, one of the most popular products containing talcum powder, is linked to increasing a woman's risk of ovarian cancer if she uses it regularly in the genital area. In a few cases, the cancer tissue was studied using an electron microscope and was found to have talc in it, which supported the claim that the cancer was caused by the body powder and increases the talc-related cancer risk.
Currently, there are more than 17,000 pending cases against J&J, and all the trials have been postponed due to the ongoing COVID-19 pandemic.
Arguments On Damages Of First Roundup Verdict On June 2

A California appeals court looks ready to uphold the nation’s first Roundup verdict but will likely reduce the $78.5 million judgment, which favored the plaintiff as the court found the company guilty of not providing necessary guidelines to the users about the weedkiller.
A former California school district groundskeeper filed the lawsuit against Monsanto on January 28, 2016, alleging that he developed non-Hodgkin’s lymphoma because of the overexposure to glyphosate present in Monsanto’s Roundup. In August 2018, a San Francisco Superior Court jury settled the lawsuit in the plaintiff's favor by rewarding him $289 million in damages, which was later reduced to $78.5 million.
Lawyers representing Monsanto had argued to reverse the verdict or to start a fresh trial as it lacked evidence. The company even wanted the court to reduce future economic damages and wipe out the punitive damages. On the other hand, the plaintiff appealed to the court for the full settlement amount of $289 million.
As per the Appellate Courts Case Information, the United States Court of Appeal scheduled the hearing of the case to June 2, which will focus on the discussion of whether the amount of the damages provided to Johnson is appropriate.
IARC, considered to be the apex in the field of cancer research, classified glyphosate as a “probable human carcinogen.” According to IARC, Roundup is made up of other ingredients that are toxic in themselves, and are also known to increase the toxicity of glyphosate. Monsanto has known this for many years but still refuses to study the link between cancer and Roundup.
Monsanto has a brief history of legal troubles and Glyphosate is just another herbicide of the company to attract lawsuits. Plaintiffs across the U.S. have filed numerous lawsuits. A plaintiff from one of the Roundup lawsuits claims that she worked as a grower’s assistant on a crop field in New York from 1994 to 1998 where Roundup was regularly sprayed indoors and outdoors resulting in chronic lymphocytic leukemia in 2012. She eventually quit the job and is currently seeking reasonable compensation and punitive damages in court.
Bayer acquired Monsanto in 2018 and currently faces more than 52,000 Roundup cancer lawsuits. The company has lost all the cases so far and has paid around $191 billion combined in damages.
