Pittsburgh Gets $7.8M to Enhance Opioid Care

The University of Pittsburgh School of Pharmacy's Program Evaluation and Research Unit (PERU) has been awarded a substantial five-year, $7.8 million grant from the National Institute on Drug Abuse.
The grant aims to enhance the quality of care provided to patients dealing with opioid use disorder (OUD) throughout Pennsylvania. This initiative will establish the Helping to End Addiction Long-term (HEALing) Measures Center at the University of Pittsburgh. The primary focus of this center will be the development and implementation of measurement-based care in 20 community opioid treatment programs across Pennsylvania. The overarching goal is to improve treatment accessibility, encourage recovery, and reduce fatal overdoses throughout the state.
While opioid overdose deaths in Pennsylvania saw a decrease from 2021 to 2022, OUD and other substance use disorders remain significant public health challenges. Medications such as buprenorphine, methadone, and naltrexone are crucial components of OUD treatment. However, a substantial portion of patients, approximately 20%, discontinue treatment within the first month, with up to 80% discontinuing within the initial six months.
Researchers believe that even a small reduction in medication discontinuation rates could translate into saving thousands of lives. The key challenge lies in ensuring that patients receive appropriate treatment at the right time and that the quality of care is sufficiently high to facilitate stable recovery.
PERU, in collaboration with the Pennsylvania Department of Human Services' Centers of Excellence for Opioid Use Disorder, will expand on an existing partnership to integrate measurement-based care into current treatment practices and workflows. This model, commonly applied in physical and mental health care settings but not previously implemented in opioid treatment programs, involves systematically evaluating patients' symptoms to guide personalized care based on their evolving conditions.
The project will assess implementation effectiveness, cost-effectiveness, and clinical effectiveness, including reductions in patient symptoms and improved treatment retention. These insights will inform strategies to enhance treatment efficiency and effectiveness and provide a blueprint for scaling measurement-based care to additional opioid treatment sites.
The HEALing Measures Center will collaborate with researchers from Northwestern University Feinberg School of Medicine and (RTI) International, along with community partners at the Pennsylvania Department of Human Services, the Pennsylvania Department of Drug and Alcohol Programs, UPMC, and the Community Care Behavioral Health Organization.
Manufacturers Seek Dismissal of Delaware Zantac Lawsuits

A Delaware judge is currently deliberating on the fate of thousands of Zantac lawsuits, assessing whether they will proceed in state court following a prior federal dismissal.
Zantac, or ranitidine, a popular heartburn and acid reflux medication, was withdrawn from the market in 2019 due to the discovery that its active ingredient is inherently unstable, generating high levels of N-Nitrosodimethylamine (NDMA), a known carcinogen.
In the past four years, major pharmaceutical companies like GlaxoSmithKline, Boehringer Ingelheim, Pfizer, and Sanofi have faced Zantac lawsuits from former users who allege various cancers, attributing them to NDMA exposure. Initially centralized in federal court for coordinated proceedings, a controversial 2022 ruling excluded all plaintiffs' expert witnesses under federal evidentiary rules, resulting in the dismissal of federal Zantac lawsuits.
Appeals challenging the federal ruling are underway, but its impact is limited to federal cases, allowing Zantac lawsuits in state courts, including Delaware and California, to continue. In Delaware, a judge is now considering manufacturers' arguments to dismiss over 70,000 lawsuits, claiming they lack scientific support. The outcome of these hearings may dictate the future of Zantac litigation in the state, mirroring the federal situation. If the judge follows a similar standard and excludes expert witnesses, it could spell the end of the litigation.
While GlaxoSmithKline reached a settlement in November, resolving the first California state trial and three other slated trials, these outcomes wouldn't set legal precedents for other pending lawsuits. The trials were seen as indicators of how juries might respond to evidence and testimony, with the potential to influence tens of thousands of nationwide trials.
The Delaware judge is expected to apply similar legal standards as the federal court but may reach a different conclusion. Unlike federal cases, Delaware employs different expert witnesses, introducing a unique dynamic. The ongoing proceedings underscore the complexity of Zantac litigation, with the potential to impact thousands of claimants seeking accountability for alleged cancer-related injuries due to the now-recalled medication.
The decision in Delaware will be pivotal, determining whether these cases advance and providing insights into the evolving landscape of Zantac litigation at the state level.
Opioid Exposure in Womb Tied to Infections, Skin Issues
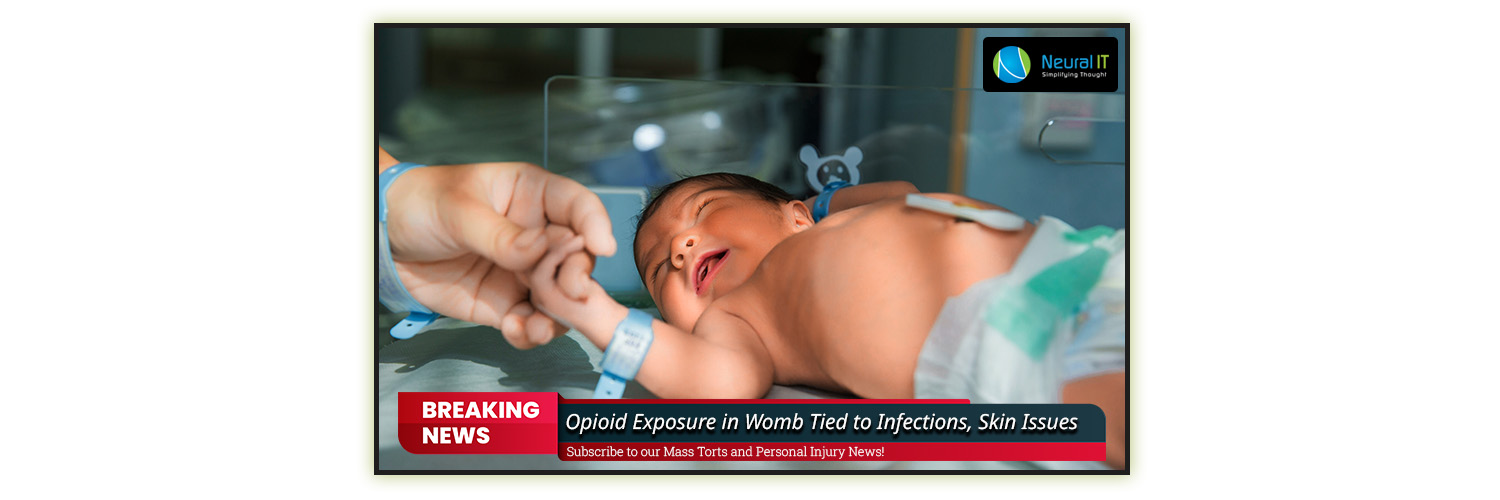
A recent study suggests that exposure to opioid pain medications during pregnancy may elevate the risk of severe infections and other health issues in children, both before and after birth.
The research, conducted by the University of Western Australia and Johns Hopkins University School of Medicine, indicates that infants exposed to opioids in utero are more prone to immune-related health problems. These issues include infections, asthma, eczema, allergic reactions, both at birth and before the age of five.
Previous studies have established a connection between opioid use during pregnancy—such as codeine, fentanyl, and oxycodone—and serious, potentially life-threatening health conditions or birth defects in newborns. Infants exposed to these potent painkillers before birth are also more likely to face a higher risk of mortality before reaching one year old.
While exposure to opioids and subsequent withdrawal has been shown to suppress the immune system in adults, limited data exists on how prenatal opioid exposure affects the immune system of unborn babies and whether it leads to immune-related diseases in childhood.
The researchers analyzed data from 401,462 babies born in Western Australia between January 1, 2003, and December 31, 2018. Of these, 1,656 were exposed to two or more opioid medications before birth. The study followed up with participants yearly for nearly five years to assess the impact of prenatal opioid exposure on their health.
The findings revealed that prenatal opioid exposure was associated with increased risks of various infections, including respiratory, neurologic, eye, digestive tract, skin, soft tissue, and viral infections, as well as eczema and dermatitis, compared to those not exposed. Infants exposed to opioids before birth also experienced neonatal abstinence syndrome (NAS), a severe condition where infants born to mothers with drug addiction undergo withdrawal symptoms, leading to an increased risk of infections and skin conditions.
The data further indicated that those exposed to opioids before birth faced a higher risk of developing severe asthma requiring hospitalization before the age of five, compared to those who were not exposed. Infants exposed to opioids prescribed for opioid use disorder had a higher likelihood of developing childhood eczema or dermatitis, while those exposed to opioids prescribed for pain had an increased risk of yeast, urinary tract, conjunctivitis, and sepsis infections.
Past research has established that infants exposed to opioids before birth are at risk of being born with debilitating birth defects and other serious health conditions. A recent study linked a new syndrome to prenatal fentanyl exposure, causing exposed babies to be born with cleft palates, clubbed feet, and other birth defects similar to those seen in Smith-Lemli-Opitz syndrome, a developmental disorder causing learning disabilities and physical birth defects.
Neonatal abstinence syndrome, resulting from opioid exposure before birth, has led to legal actions against drug manufacturers and distributors due to the severe side effects experienced by infants exposed to these medications during pregnancy.
Washington settles with J&J for $150M in opioid crisis

The Washington state attorney general has declared a settlement of $149.5 million with pharmaceutical giant Johnson & Johnson, concluding a legal battle initiated over four years ago regarding the company's involvement in the opioid addiction crisis.
This announcement coincided with a troubling trend in opioid overdose deaths, which more than doubled from 2019 to 2022, reaching 2,048 deaths in the latter year, as per the latest data from the Washington State Department of Health.
The attorney general expressed that the repercussions of this harm are now a challenge for policymakers to grapple with, or for families and individuals who deal with the real tragedy of addiction in a profound and different way.'
The settlement agreement is contingent on approval from a judge. If sanctioned, the deal would allocate over $20 million more to address the opioid crisis compared to the state's participation in a national settlement with Johnson & Johnson in 2021, according to the attorney general’s office.
Since the 2000s, various entities including drugmakers, wholesalers, pharmacy chains, and consultants have collectively agreed to pay more than $50 billion to state and local governments to settle claims implicating their role in fueling the opioid crisis. These settlements are predominantly intended to fund initiatives combating the nation’s addiction and overdose crisis.
From 1999 through 2021, drug overdoses caused over 1 million deaths in the U.S., with opioids being the major contributors. The crisis initially revolved around the acceptance of prescription painkillers in the 1990s and later shifted to heroin. Over the past decade, synthetic opioids like fentanyl, present in the supply of many street drugs, emerged as the primary culprits behind the soaring death toll.
In 2020, Washington state’s Democratic attorney general filed a lawsuit against Johnson & Johnson, contending that the company played a role in the pharmaceutical industry's expansion of prescription opioids. The lawsuit alleged that the company significantly impacted Washington’s opioid crisis by misleading doctors and the public about the efficacy of opioids for chronic pain and the risks of addiction.
Highlighting Johnson & Johnson's involvement, the attorney general's office pointed out that in 2015, the company was the largest supplier in the country of the active pharmaceutical ingredients used in opioid drugs.
Johnson & Johnson responded with a written statement asserting that its fentanyl patch, Duragesic, and its opioid Nucynta collectively accounted for less than 1% of opioid prescriptions in both the state and the U.S. The company clarified that it has not sold prescription opioid medications in the country for several years, and it maintained that its actions concerning the marketing and promotion of these prescription opioid medications were appropriate and responsible.
If the settlement is approved, the funds will be distributed by the end of the fiscal year. This timeline enables the Legislature to allocate the money during the ongoing legislative session. Half of the settlement amount will be directed to a state account, while the other half will go to an account designated for local governments, according to the attorney general’s office.
This settlement comes about two years after the nation’s three largest opioid distributors agreed to pay Washington state $518 million, with the majority of the funds aimed at addressing the addiction epidemic.
